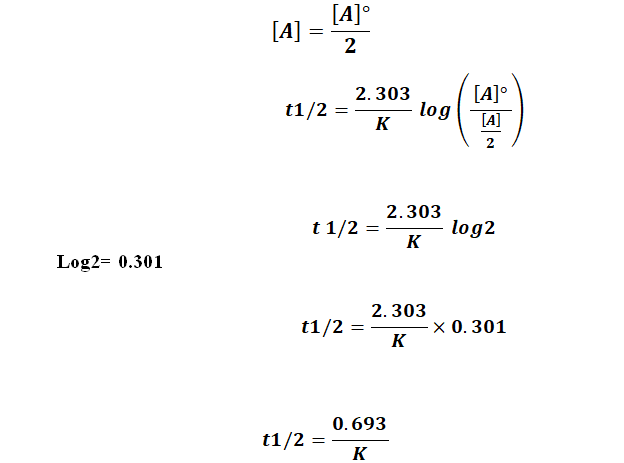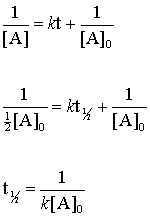half life formula for zero order reaction
The half-life of the reaction is denoted by t 12 and is expressed in seconds. For a zero-order reaction the half-life equation is given as.

Solved Half Life Equation For First Order Reactions T 2 Chegg Com
The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction is given as t12 0693k The half-life of a second-order reaction is given by.
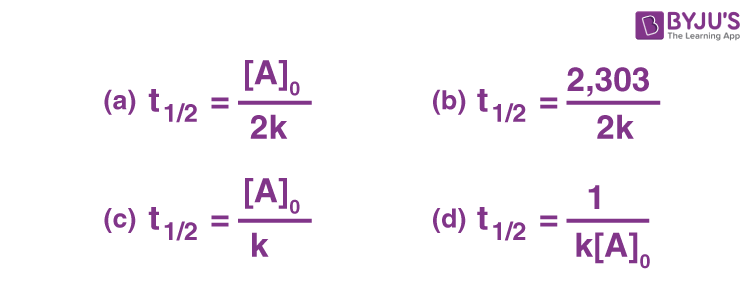
. A A 0 k t AA_0-kt A A 0 k t. The half-life period of first and zero order reaction can be calculated using the integrated rate equation-. Graphical relations and half lives.
A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant. For zero order reaction. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a.
Determining a half life. Equations for Half Lives. For a zero order reaction A products rate k.
A A_0 kt which is the required equation. This becomes evident when we rearrange the integrated rate law for a first-order reaction to produce the following equation. For a zero order reaction k frac left Rright_ 0-left Rright t k tR0 R At t t_ 12quadleft Rrightfrac 1 2left Rright_ circ t12 R 21 R.
The integrated rate law for the zero-order reaction A products is A_t -kt A_0. T ½ A o 2k For a. T_12 is a timescale in which each half-life represents the reduction of the initial population to 50 of.
From the above-integrated equation we have. Now replacing t with half-life t12 in. For a general reaction.
The time taken for the concentration of a given reactant to reach 50 of its initial. A A 0 - kt. Relationship Between Half-life and Zero-order Reactions.
The rate equation for zero order reaction. For a first zero order. Converting a half life to a rate constant.
Half-Life for a Zero-Order Reaction The integrated rate law for a zero-order reaction is given by. Substituting A 02 for A. Half-Life of a Zero Order Reaction.
A_t ktA_0 For a half-life tt_frac12. T 12 R 02k From the above relation we can say the Half-Life of a zero-order reaction is directly proportional to the initial concentration of the reactants and inversely. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k.
451 ln A 0 A k t. Remember the half-life of a reaction changes with the order of the reaction. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.
The half-life formula for a reaction depends upon the order of a reaction. As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law. It is to be noted that the formula for the half-life of a reaction varies with the order of the reaction.
Half life formula for nth order reaction. The half-life of a zero-order reaction the formula is given as t 12 R 02 k The half-life of a first-order reaction is given as t 12 0693k The half-life of a second-order. Given below is the half-life of a zero.
For the first-order reaction the half-life is defined as t 12 0693k.
Chm 112 Kinetics Practice Problems Answers

How To Calculate Half Life For Zero Order Reactions Youtube

Determine The Half Life Of A Zero Order Reaction Youtube
Order Of Reaction Zero Order And First Order Science Vision

Zero Order Reaction Definition Examples Formula

Zero Order Reaction Definition Derivation Graph Examples

Using The First Order Integrated Rate Law And Half Life Equations Worked Example Video Khan Academy
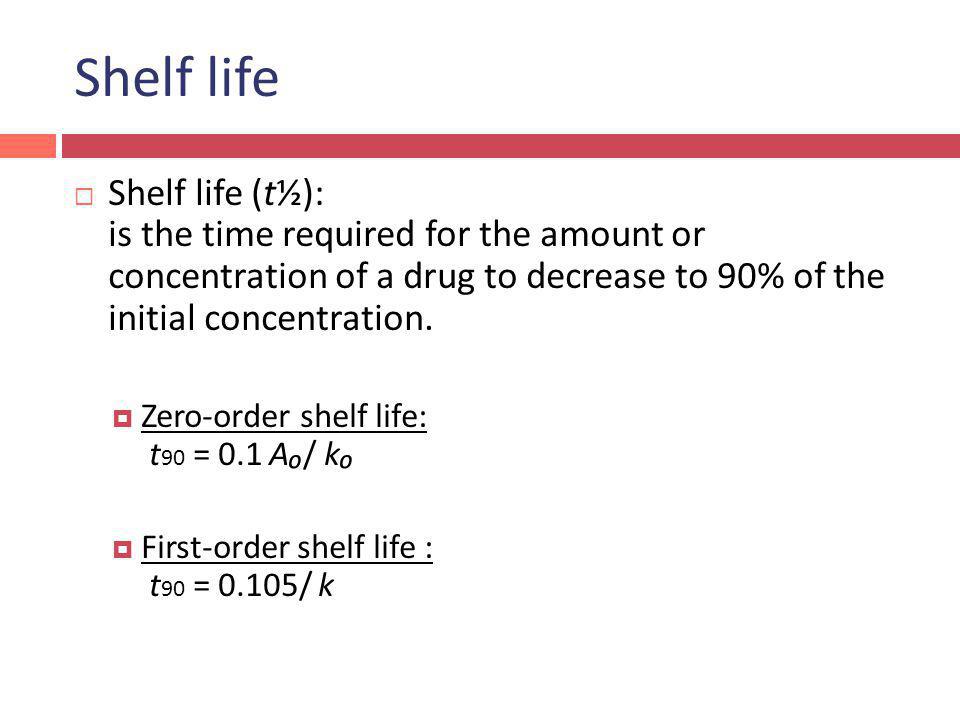
Kinetics Order Of Reactions Ppt Video Online Download

Half Life Expressions Chemistnate
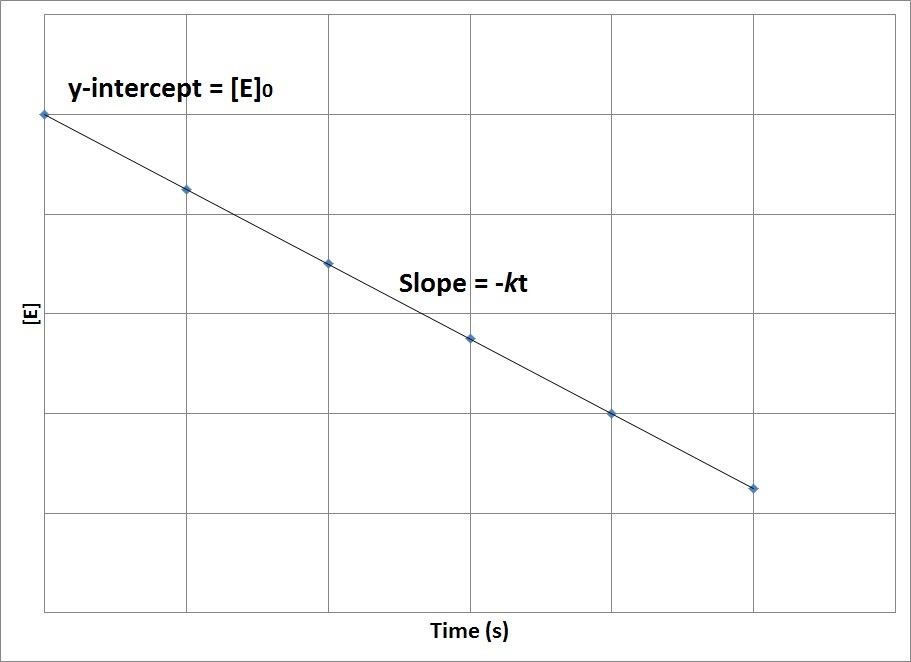
Concentration Time Relationships Integrated Rate Laws Introductory Chemistry 1st Canadian Edition

First Order Reaction Overview Equation What Is Rate Law Equation Video Lesson Transcript Study Com
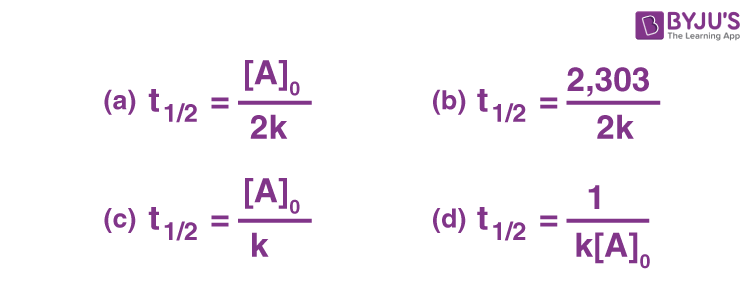
Zero Order Reaction Questions Practice Questions Of Zero Order Reaction With Answer Explanations
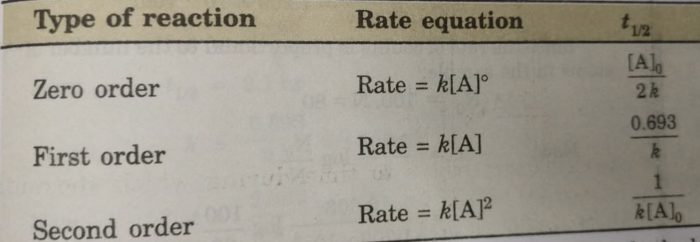
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12
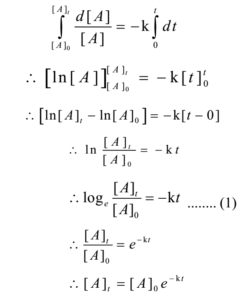
Rate Of First Order Reaction Meaning Of First Order Reaction Integrated Law